What are Particles, Ions and Radicals. How are they formed in the atmosphere?
Particles:
Particles are present in the troposphere. They vary in number from several hundred per cubic centimeter in pure air to more than 105 per cubic centimeter in polluted air. The particles also vary in size (0.1 — 10)µ Their mass ranges from 10µg/m3 in clean air to 60 — 2000 µg/m3 in polluted air.
Particles of colloidal size in the atmosphere are known as aerosols. Aitken particles are also aerosols of natural origin having diameter less than 0.2µ. Such particles originate from vegetation. Bacteria, fog, pollen grains and volcanic ash are other particles of natural origin in the atmosphere. In case of particles, their size is more important than their total count.
Particles in the size 0.1-1µ are responsible for the electrical phenomena in the atmosphere. They are also responsible for cloud and fog formation. Besides, particles serve as nucleic for the formation of ice crystals and water droplets. The particles of metals also have catalytic effects on oxidation and photo-chemical oxidation’s. Finally, particles play an important role in determining the heat balance of the earths atmosphere via light reflection.
Formation of Particulate Matter:
Inorganic particulate matter originates from the following sources:
- Automobile exhaust discharge lead particles is the atmosphere. It is estimated that in USA more than 2 X 105 tonnes of lead are dumped in the atmosphere every year. However, with switching off to unleaded petrol, this quantity is decreasing over the years.
- Combustion of pyrite containing coal dumps besides carbon particles in the form of ash also metal oxides.
3FeS2 + 8 O2 →Fe3O4 + 6SO2
The formation of aerosols is explained by the atmosphere oxidation of SO2 to SO3, followed by reaction with moisture to produce H2SO4. The acid produced reacts with basic air pollutants like NH3. or CaO to form salts
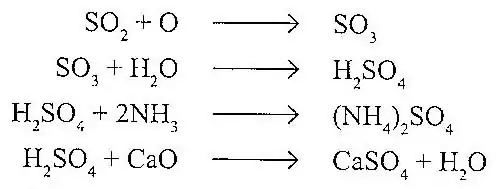
In a similar way the oxides of nitrogen react with moisture to produce HNO3. The acid produced reacts with basic air pollutants to produce nitrates.
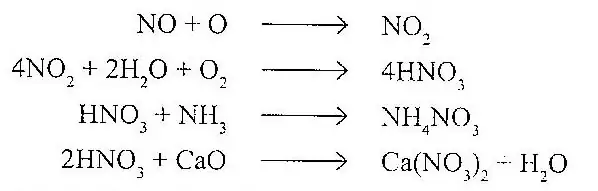
The net result is the formation of aerosol mist consisting of droplets containing salts of H2SO4 and HNO3.
A typical analysis of particulate matter in the air in united states urban locations revealed the following composition (µg/m3), total suspended particulate matter (105), NH4+ (1.3), NO,— (2.6), SO42 (10.6), benzene soluble organics (6.8), Sb (0.01), As (0.02), Cd (0.002), Cr (0.015), Cu (0.09), Fe (1.58), Pb (0.79). Mn (0.10), Ni (0.034), Sn (0.02), Ti (0.04), V (0.05) and Zn (0.07) .
Organic particulate matter:
Is believed to originate from a variety of source like emissions from vegetation and automobiles, combustion of fuels etc. Such particles are in 1 µ size range and are potential health hazards. For example, polycylic (polynuclear) aromatic hydrocarbons (PAH) in organic particulate matter have carcinogenic effect. One such compound responsible for carcinogenic effect is beno (∝-) pyrene. Such compounds generally occur in urban atmospheres at about 20µg/m3 level and originated from the pyrolysis of paraffins present in fuels and plant material.
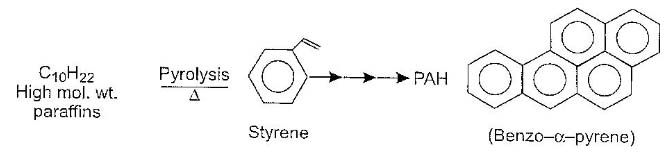
The PAH formed remain adsorbed on soot particles, which are formed as a residue on combustion of fuels in automobiles and electricity generating power plants, which use coal as the raw material. Generally speaking, the soot particles, which we come across contain adsorbed PAH and toxic metals. A typical soot particle is represented is shown in below figure.
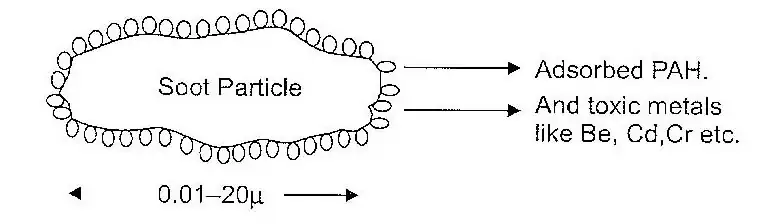
The atmospheric particles (soot particles) enter human body through respiration. These get lodged in the lungs causing health hazards. The removal of particulate matter from the gases is important for controlling air pollution.
Ions and Radicals:
Ions are atoms that have either lost one or more electrons, making it positively chamed (or cations) or gained one or more electrons making it negatively charged (anions). The ions are normally present in the ionosphere region of the mesosphere. In this region, positive ions like O2+, O+, NO+ etc. and electrons exist at significant levels. These species are formed due to solar radiations. During the night, the UV radiations are not available and so these ions recombine with free electrons to give neutral species from which they originated.
The ions are influenced by the earths magnetic field. This results in the formation of two belts of ionizing particles encircling the earth. These are known as van Allen belts.
Radicals:
Radicals are a group of atoms, either in a compound or existing alone. Free Radicals are atoms or group of atoms with an unpaired valence electron. Free radicals can be produced by photolysis or pyrolysis in which a bond is broken without forming ions. Because of their unpaired valence electrons, most free radicals are extremely reactive. Besides ions, the atmosphere also contains free radicals, which take part in chain reactions in which one of product is a free radical. The chain can be terminated by combination of two free radicals.
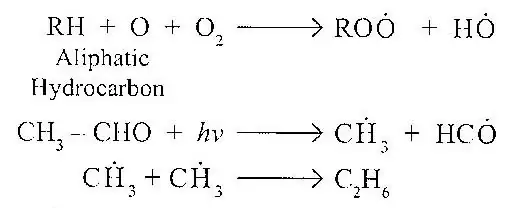
Free radicals play an important role in photo-chemical smog formation. This aspect forms the subject matter of a subsequent section.


